Fail early
Have a look at the article in Chemical and Engineering News "Drug Discovery - Filtering Out Failures Early in the Game", June5, 2000 C&EN page 63. This article is not availabe on the Internet, but more recent ones very similar to it. Search Google with "Drug Discovery - Filtering Out Failures Early in the Game".
This articles gives a pretty good overview about the different efforts of drug and software companies to develop methods to weed out early the "false negatives". "These are drug candidates that give no hint of having undesirable qualities until they are in the final stages of testing, when they're found out to have safety issues."
At Pfizer, some of the primary reasons for attrition of drug candidates are safety, pharmacology and poor ADME characteristics.
The ratios are shown below:
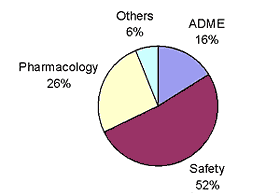
In terms of poor ADME characteristics, clearance is the dominant cause of attrition; the ratios documented at Pfizer are: poor absorption-20%, poor clearance-64% and poor distribution-16%.
Historically (before 1995), attrition of new chemical entities due to pharmacokinetics was almost 20%. Recently, from 1997 to 2001, pharmacokinetic-related attrition was slightly over 10%. This decrease has been attributed to better screening and identification of drug metabolism problems earlier in the discovery process.
from: Influencing Chemists to Minimize Attrition,Jae Lee, Pfizer Inc. - 2002
The methods that are mainly used for ADMET
databases:
- Reaxys
- SciFinder
- Metabolite
- Toxicity Database
- xPharm, one of the few sources with pharmacological data
and in silico methods:
Here are the features of some databases:
Toxicity |
Metabolite |
Reaxys |
| Data from 1902 onwards from the RTECS database includes journal abstracts, U.S. federal standards and regulations, government reports, and unpublished EPA test submissions. | Data from original literature covering 1901 onwards abstracted from: Biotransformation von Arzeneimitteln (S. Pfeifer and H. Borchert ed. ) published 1977-1983; Pharmacokinetics (S. Pfeifer and H. Borchert ed.) published 1986 -1990; ongoing semiannual updates 1991 - present from 49 leading journals and FDA NDAs. | The Reaxys database alsocontains pharmacological, toxicological and ecological data. |
Toxicity data in six categories:
|
Graphically searchable
biotransformations and metabolic schemes from in vivo and in
vitro studies. Includes information on
|
Beilstein covers the
following data:
|
| Data is abstracted from over 2,950 worldwide sources, including: scientific journals, conference proceedings, symposia reports, reviews, and more. Covers presently 145,035 compounds. | Covers over 7,900 parent compounds -including drugs, agrochemicals, industrial chemicals and environmental contaminants. | EcoPharm adds more than 145'000 records per year from 180 key journals. |
| Links to chemical structures included in the Metabolite database, and access to original journal articles (through LitLink/Standard or LitLink/Pro Server). | Links to chemical structures included in the Toxicity database, and access to original journal articles (through LitLink/Standard or LitLink/Pro Server). | Access to original journal articles (through LitLink/Standard or LitLink/Pro Server). |